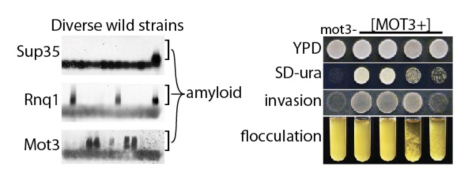
Prions are unusual proteins that have the capacity to change shape and to selectively template that change in shape to other proteins of the same type, a property shared by prions and other amyloid-forming proteins across the evolutionary spectrum. While amyloids can cause devastating economic hardship in an extraordinary variety of settings, we have found that, surprisingly, prions can also be beneficial. In yeast, prions, rather than being toxic can help give cells new capacities to survive and thrive. In doing so, they have the capacity to act as drivers of evolutionary novelty by serving as beneficial “bet-hedging” mechanisms in the wild to enhance survival of fluctuating environments.
On the other hand, amyloids contribute to degenerative diseases of our aging population, and to the production of infectious bacterial biofilms that resist eradication by antibiotics, bacteriophage and even bleach. Research in our lab takes advantage of the remarkable methodological approaches yeast cells offer for the study of difficult problems in protein folding and assembly and capitalizes on technological innovations recently developed in our laboratory for structural and mechanical investigations of amyloid proteins. The model protein for much of this work is Sup35, a translation termination factor of yeast. It has provided a wealth of information on nucleation and assembly pathways in vitro, while in vivo, strains of the prion with defined phenotypic consequences and patterns of inheritance have been intensely investigated. In recent years dozens of new yeast prions have been discovered by our lab and others. By focusing on this class of proteins, proteins with completely unrelated amino acid sequences and diverse biological functions, we aim to uncover fundamental insights on the assembly of these proteins that will be applicable to a variety of problems that currently cause great suffering and economic hardship.